|
|
|
| Equipment | LSM710 | Multiphoton STED | Spinning Disc Confocal | DIMs | PALM | FCS | FLIM | TEM | Micro-Injection | HCS | Workstataions and Tools |
Super resolution (STED) and multiphoton microscope
This is a complex system with multiple capabilities. It is:
1) A super resolution microscope with STED (STimulated Emission depletion) which is capable of obtaining fluorescence images with resolution down to <50nm.
2) A multiphoton microscope with tunable laser from 680-1064nm.
3) A Fluorescence Lifetime Imaging system based on time domain for FLIM measurements.
4) A Fluorescence correlative Spectroscopy system for detecting molecular associations.
5) A combination of optics and software module capable of obtaining fluorescence images down to ~120nm.
STED uses a donut shaped depletion laser to modify the point spread function of the beam down to sub-diffraction limit level to achieve super resolution microscopy at resolution below 50nm (STED principle link). The system is equipped with 594nm, 660nm and 775nm depletion lasers for variety of dyes. For some details system information, please visit this website: Leica STED.
Multi-photon microscope uses an ultra-fast pulsing laser to excite fluorescent molecules. Briefly, 2 (multi)-photon microscopy is based on the principle that a given fluorescent molecules which normally would only be excited by absorbing the energy of a single photon of certain wavelength can also be excited by combination of the energies produced by simultaneous absorption of 2 (or multi-) lower-energy photons of double (multiple) wavelength. The emission of the fluorescence is quadratically dependent on the excitation intensity. This steep dependence of absorption rate on photon concentration gives multi-photon Laser Scanning microscope the intrinsic three-dimensional resolution with the depth of field defined by a) intensity of the excitation light, b) numerical aperture of the lens used and c) the wavelengths of the lights. This z-resolution is comparable to conventional Laser Scanning confocal microscope. . |
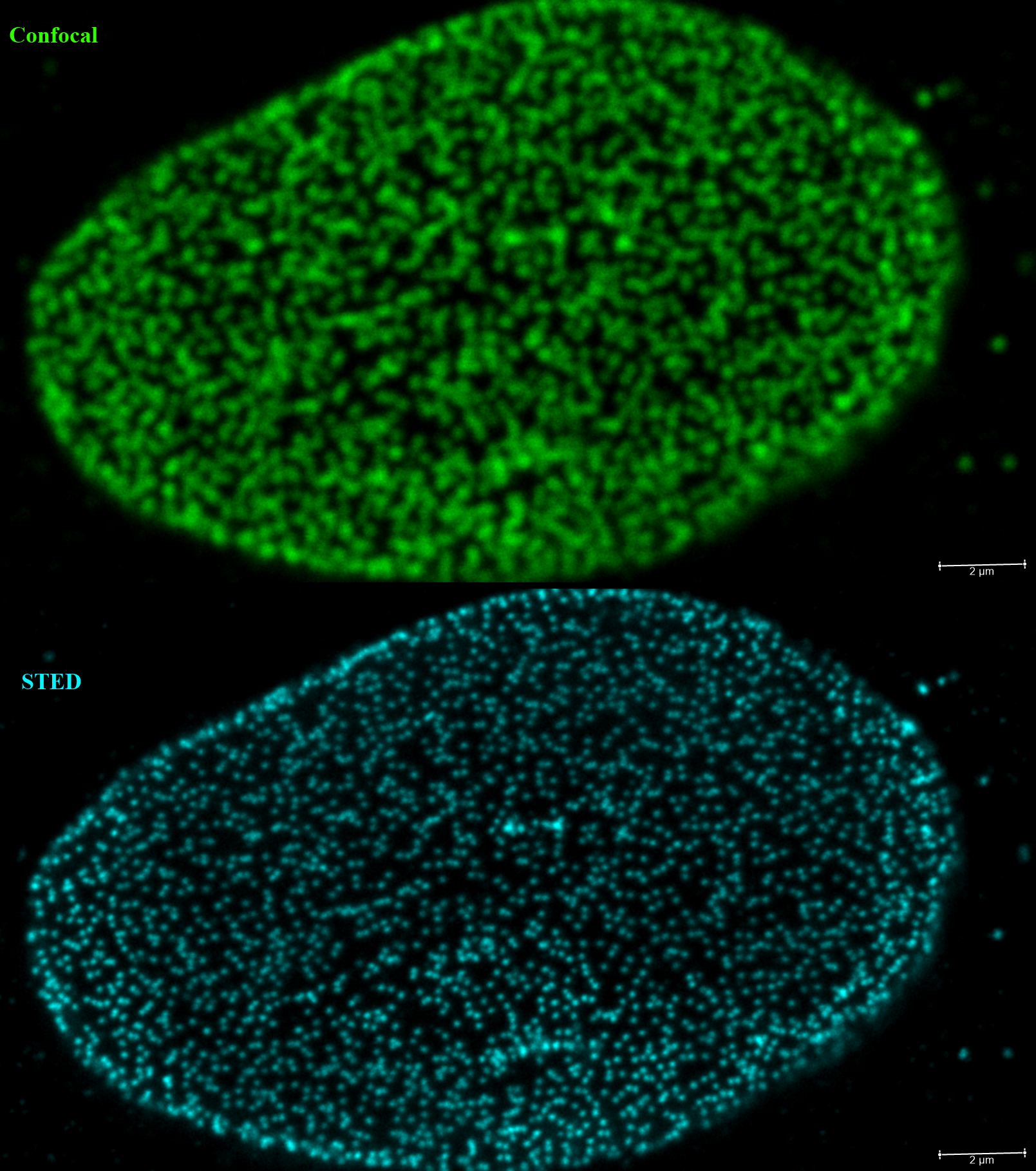
COS cells were stained with an anti-nuclear pore protein conjugated to Star635P dye and imaged with Confocal (top) and STED (bottom) at the same optical plane to demonstrate the improvement in resolution with STED (scale bar 2um). |
Multi-photon offers some distinct advantages over confocal microscope:
- Longer wavelength used for excitation allows imaging deeper into thick specimens due to less scattering of longer wavelength (IR) light.
- Photo-bleaching and damaging are confined to the focus points only.
- It is possible to image UV dyes with infra-red light. This is particularly useful for live cell imaging where UV light is highly damaging to the cells
Therefore, multi-photon microscopy is highly suitable for imaging of live, thick specimens.
The system is also capable of measuring fluorescence life time with the equipped pulse lasers. The system is equipped with a white laser which is pulsed and could be tuned continuously from 470-690nm in addition to the IR laser with tuning range of 680-1024nm. In addition, the system is able to perform Fluorescence correlative spectroscopy, deconvolution and high speed imaging.
The system is a Leica Falcon SP8 system with STED (Leica_Falcon) with a Spectra-physics femto-second laser (Insight, Newport Inc.). With the following optics:
|
Leica SP8 Falcon STED info:
Microscope: Leica SP8 (inverted )
Objectives:
5x 0.15NA HC Plan-Fluotar
10x 0.4 NA HC Plan Apo
25x 0.95NA Water HC Fluotar
86x 1.2NA Water HC Plan-Apo
100x 1.4NA Oil Plan-Apo
Laser lines
Ti:Saphire: Dual beams with fixed at 1064nm and tunable 680-1064nm
White Laser II: continuously adjustable wavelength from 470 to 690nm
Argon: 458, 488, 514nm
Diod: 405nm
Detectors
2 PMTs, 3 Hybrid detectors, 1 transmission PMT
|
 |
|
|
